- Air Homepage
- Upper Atmosphere
- Grams Per Liter
How Grams per Liter Explain Concentration and Density
There's a density to everything on earth. Density is measured in grams per liter (g/L) or other units of mass per volume. You might become concerned...
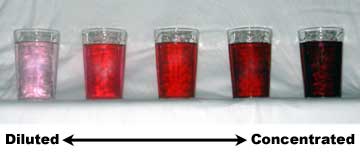
Concentration is the amount of a substance (good or bad) in your immediate vicinity. A substance's density is its mass per unit volume and density goes up when there's a higher concentration of a substance in your space.
These concepts are not only closely related, they're important. Concentration in chemistry is how much of a substance is in a given volume of a solution. Many chemical reactions and processes depend on the concentration of a particular substance, as it determines how effective the reaction will be or how toxic it will be.
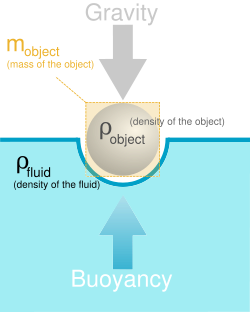
In physics, density describes how much matter is in a given volume. It's possible to calculate a lot of physical properties using density, like the weight of an object, the buoyancy of a material, and the strength of various materials.
There are a lot of contexts in which people care about concentration and density in everyday life. Understanding the concentration of ingredients in a recipe can help you get the right taste and texture. Also, farmers measure soil density to figure out how much nutrients and water their crops need. Here are some everyday examples:
It's about the same mass as a paper clip or a third of a sugar cube, and a liter is about the same size as a quart.
At sea level, a liter of air
weighs about one gram, and less at higher elevations. There are about 1000 grams in a liter of water.
Here are some examples of density, also called specific gravity.
Here are a few more. The weight of wood varies from 200 grams per liter to over 1000 grams per liter. It floats if it's less than a thousand.
Ice weighs 920 grams per liter. It floats.
There's a lot of weight in metal. Aluminum is 2700, gold is over 19,000, and copper and steel are 8000 to 10000.
They're also pretty dense, with most weighing between 2500 and 3000 grams per litre. Glass is made from rocks, too and weighs about the same.
Things that affect grams per liter
As most things expand when heated, the density of most liquids and solids depends on the temperature. They'd get bigger without changing their mass.
Gas density changes a lot with pressure and temperature. Thermodynamics thermodynamics
says that doubling the density of a gas will double its volume. It's Boyle's Law applied to ideal gases. Temperature and pressure will also go up. How much? It's harder to calculate than the change in grams per liter.
What's the density of gasoline? Over 100 hydrocarbons and minerals make up this stuff, each with its own density. Every tank could have a different mix, so the density would be different. It's usually between 700 and 800 grams per liter of petrol.
For nearly all my unit conversions, I use this free website: OnlineConversion.com You can convert just about anything. Click "density" to see all the options. Try it out and see if it helps.
Now what about concentration?
In liquids, grams per liter (g/L) measure the concentration of a substance. You can use this to determine how much of a substance is in a given amount of liquid.
Densities are really concentrations, or is density really a concentration. It's both a way of expressing how much something is spread out.
The rest of that volume is filled with a solvent, like water or air. In some cases, we use grams per liter to indicate both concentration and density.
When you dissolve your sugar cube in one liter of water by mixing it really well, you end up with three grams of sugar per liter. You should be able to taste it. Sugar is distributed evenly throughout the liter in this concept of concentration.
However, it depends on the solvent. In this case, the water needs to keep the sugar particles off the ground and the bottom of the container. The air might not do that as well. The sugar powder would slowly settle out.
As part of air quality modelling, we measure dust concentrations in grams per liter, which are solid particles suspended in the air. Even if they settle out eventually. In that case, we can calculate the rate of deposition in grams per square metre per hour. We can use similar, smaller units for concentrations, like micrograms per cubic meter.
Total suspended particulates (TSP) or just particulates are what we call airborne dust. I predict these concentrations in the air in my full-time work.
Gases in the air don't settle and are removed by other means. Plants or condensation can remove carbon dioxide or water vapour from the atmosphere indefinitely.
Concentrations are also given as fractions of the total mass or volume of the solvent (air or water). Sometimes you hear percent (%), parts per million (ppm) or parts per billion (ppb).
Generally, relative concentrations (using % or ppm) help us make recipes with the right amount of sugar, flour and other stuff, figure out how strong an alcoholic drink is or whether you have too much in your blood (another solvent) to drive, and figure out whether fumes are deadly poisonous or explosive.
Concentration tells you how strong a vapour is, how humid it is outside, and how salty the ocean is. There's one exception, though: relative humidity. It's not the percentage of the total volume occupied by water vapor in this case, even though it's given as a percent. In technical terms, it's the fraction of the total concentration (vapour pressures) that can stay in the parcel of air under current conditions.
Density is measured in kilograms per cubic metre, which is equal to grams per liter in SI. Since this system was invented by the French, it's spelled grams per litre.
The same goes for "metre."
What Does Grams per Liter Mean? Are You Talking About Concentration or Density?
Concentrations are expressed as percent, parts per million, or grams per liter, depending on whether you are using air or water. The same principle applies to density.
Do you have concerns about air pollution in your area??
Perhaps modelling air pollution will provide the answers to your question.
That is what I do on a full-time basis. Find out if it is necessary for your project.
Have your Say...
on the StuffintheAir facebook page
Other topics listed in these guides:
The Stuff in the Air Site Map
And,
Thank you to my research and writing assistants, ChatGPT and WordTune, as well as Wombo and others for the images.
GPT-4, OpenAI's large-scale language generation model, helped generate this text. As soon as draft language is generated, the author reviews, edits, and revises it to their own liking and is responsible for the content.



New! Comments
Do you like what you see here? Please let us know in the box below.