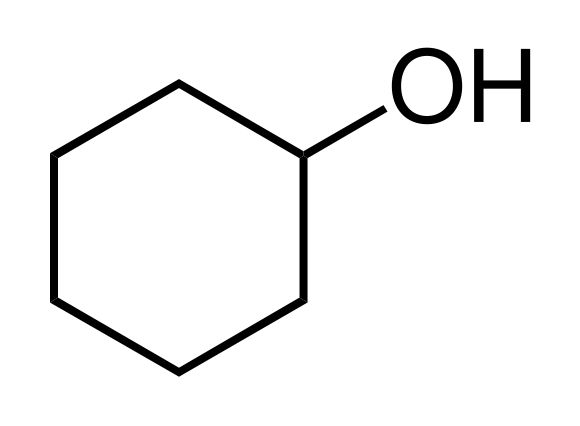
Converting cyclohexanol to to cyclohexene
by Silky Jonsteen
(Lawrence, Ks)
Cyclohexene Adventure: From Boozy Ring to Zippy Alkene! - What if you could see a simple alcohol transform into a super-reactive alkene right before your eyes, complete with bubbling reactions and that infamous rotten egg smell? Discover the magic of organic chemistry with this classic lab experiment that turns cyclohexanol into cyclohexene!
Silky says: The is an Organic elimination reaction. First we start my taking a solution of cyclohexanol, HOCH(CH2)5, which is a cyclohexane ring with an alcohol functional group attached to it. Then we add sulfuric acid (H2SO4) to the reaction. The acid will react with the alcohol group.The acid will protonate (adding a proton to an atom or molecule) the OH group, making it a H2O group. Which will then leave the cyclohexane. This will form a carbocation (carbonium ion, a positively-charged carbon atom which can attach to five other atoms, molecules or ions) and this ion is unstable. The acid which gave up a proton will abduct (grab) a Hydrogen particle and that will form a double bond in the cyclohexane, converting it to cyclohexene (which has additional bonds between the carbon atoms).
This project is useful because, a double bond is easy to break and it is more reactive than a single bond (go figure!). You can convert cyclohexene to many other products. This teaches you a basic organic reaction.
You can repeat this same procedure with other compounds that contain a leaving group. One which departs and make new compounds. Share them with your teachers, friends, family, neighbors.
The fun part about this lab was using the condenser. You had to heat up the cyclohexanol and sulfuric acid so they could react with each other. Once they reacted the newly formed cyclohexene was converted to vapor form. This new substance was condensed from the sulfuric acid and moved to a different beaker.
The heated sulfuric acid smelled bad. Like someone ate rotten eggs for breakfast, and now it's kickin' in. But on the whole, it was a fun experiment.
Barry's Response - Could have been, Silky. Any organic chemists in the crowd?
Why should someone care about Converting cyclohexanol to cyclohexene
There are several reasons why learning about this conversion is valuable:
- The conversion process from cyclohexanol to cyclohexene provides insight into chemical reactions and mechanisms. Learners can explore things like dehydration, elimination reactions, and catalysts. You can use this knowledge to understand more complex chemical reactions.
- The conversion of cyclohexanol to cyclohexene falls under organic chemistry. Students can learn about functional groups, reaction types, and organic synthesis by studying this reaction. Organic chemistry is fundamental to many fields, like pharmaceuticals, materials science, and biochemistry.
- In industry, cyclohexanol to cyclohexene conversion is important. The chemical cyclohexene is used to make plastics, solvents, and polymers. Those interested in chemical engineering, manufacturing, or related fields can benefit from understanding the process.
- The conversion of cyclohexanol to cyclohexene involves experimental techniques common in organic chemistry. Some of these methods include distillation, refluxing, and gas chromatography. These techniques can be useful for anyone interested in laboratory work, research, or pursuing a career in chemistry.
- Analytical thinking and problem-solving skills are needed to explore the conversion process. Chemical transformations involve understanding reaction conditions, identifying intermediates, and predicting outcomes. Problem-solving skills can be enhanced by engaging in such challenges.
- Students studying chemistry or related fields may need to learn about this chemical transformation. They'll be able to succeed in their studies and exams if they understand the concepts and mechanisms behind this transformation.
- Individuals with a general interest in chemistry enjoy learning about different reactions and transformations. Exploring the conversion of cyclohexanol to cyclohexene can be intellectually stimulating and personally fulfilling for them.
Here's a bit about these substance for the unfamiliar
(i.e., most of us).Cyclohexanol has the chemical formula C6H11OH. There's a faint sweet odor to this colorless, viscous liquid. It is soluble in most organic solvents and water. In the production of adipic acid, caprolactam, and nylon-6, cyclohexanol is an important intermediate.
Cyclohexanol makes adipic acid, which is used to make nylon, plastics, and other synthetic materials. Also used as a solvent in paint and varnish and a raw material for other chemicals like cyclohexanone, cyclohexylamine, and caprolactam. Besides its industrial applications, cyclohexanol has some medicinal properties and is used in some pharmaceuticals. Flavors and fragrances are also made with it.
Cyclohexene has the chemical formula C6H10. It's an unsaturated compound with a carbon-carbon double bond. It's a colorless liquid with a distinctive smell that's insoluble in water, but soluble in organic solvents. As a monomer, cyclohexene is used to make adipic acid, maleic acid, and caprolactam. Also used as a solvent for organic reactions and a starting material for other chemicals.
Cyclohexene is used to make nylon, plastics, and other synthetic materials, including adipic acid. Also, cyclohexene is used to make maleic acid, which is used to make resins, polymers, and other chemicals. Nylon-6 is made from caprolactam, another important chemical made from cyclohexene.
It's also used in the pharmaceutical industry as a starting material for certain drugs and in the fragrance industry as a flavoring agent. Additionally, it's used for polymerization reactions and extraction of natural products.
Search this site for more information now.
Comments for Converting cyclohexanol to to cyclohexene
|
||
|
||
|
||
Do you have concerns about air pollution in your area??
Perhaps modelling air pollution will provide the answers to your question.
That is what I do on a full-time basis. Find out if it is necessary for your project.
Have your Say...
on the StuffintheAir facebook page
Other topics listed in these guides:
The Stuff-in-the-Air Site Map
And,
Thank you to my research and writing assistants, ChatGPT and WordTune, as well as Wombo and others for the images.
OpenAI's large-scale language generation model (and others provided by Google and Meta), helped generate this text. As soon as draft language is generated, the author reviews, edits, and revises it to their own liking and is responsible for the content.